Objectives
The objective of this project is to utilize vagus nerve stimulation(VNS) to develop a non-invasive rehabilitation device for treating impairments associated with ischemic stroke. The device consists of an array of electrodes situated on a neck collar made from adjustable, non-conductive materials. These electrodes will release electrical pulses with a mild current intensity to stimulate the vagus nerve, thereby increasing circulation to the briain and reducing infarct volume. Electrical stimulation is verified by tracking the movement in the laryngeal neck muscle through an electromyography (EMG) device located in the center of the neck brace.
Circuit Component
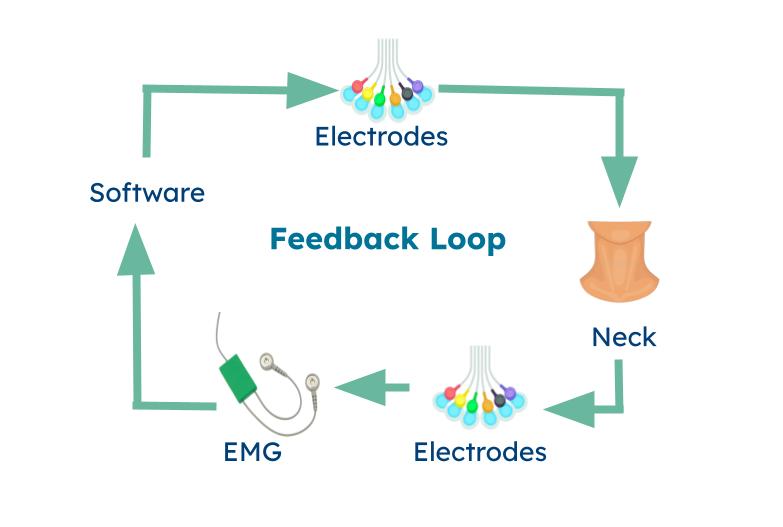
 One main component of Stingray is the circuit components that comprise the feedback loop. The feedback loop begins with the programming software, which drives the rest of the feedback loop. The software outputs a voltage, which is converted to current. The current is transmitted through electrodes connecting the hardware/software and the neck collar together. The electrodes are placed on the neck collar's interior. The electrodes emit a current onto the vagus nerve, stimulating the brain, sending a signal back through the laryngeal muscle. The electromyography (EMG) device collects this signal and sends it back to the software for analysis. If the signal from the vagus nerve is less than the preset threshold value, then the voltage output is slowly increased in increments, and the feedback loop repeats.
One main component of Stingray is the circuit components that comprise the feedback loop. The feedback loop begins with the programming software, which drives the rest of the feedback loop. The software outputs a voltage, which is converted to current. The current is transmitted through electrodes connecting the hardware/software and the neck collar together. The electrodes are placed on the neck collar's interior. The electrodes emit a current onto the vagus nerve, stimulating the brain, sending a signal back through the laryngeal muscle. The electromyography (EMG) device collects this signal and sends it back to the software for analysis. If the signal from the vagus nerve is less than the preset threshold value, then the voltage output is slowly increased in increments, and the feedback loop repeats.
Neck Collar Component
 The neck collar, made of flexible, medical-grade nylon material, houses electrode arrays on both sides of the neck to access the vagus nerve. These electrodes release electrical pulses with a mild current intensity of 1-3 milliamps (mA) of therapeutic dosage. To verify that the vagus nerve is properly stimulated, an electromyography (EMG) device located at the center of the collar, will measure the amount of electrical activity in the laryngeal neck muscle.
The neck collar, made of flexible, medical-grade nylon material, houses electrode arrays on both sides of the neck to access the vagus nerve. These electrodes release electrical pulses with a mild current intensity of 1-3 milliamps (mA) of therapeutic dosage. To verify that the vagus nerve is properly stimulated, an electromyography (EMG) device located at the center of the collar, will measure the amount of electrical activity in the laryngeal neck muscle.
Design Criteria
The design criteria are established to make the device safe, comfortable, and reliable. The neck collar fabric is composed of medical-grade Nylon 6, a material commonly used in medical devices. This material was chosen due to its flexibility and non-conductive qualities. The neck collar has a circumference of 18 inches, determined by the average adult neck size, with an acceptance criteria of 0.25 inches. The neck collar height is 3 inches to accomodate the electrode components and to secure a snug fit to the patient. The acceptance criteria for the neck collar’s height is 0.1 inches. Desired current stimulation falls between 1-3 mA in accordance with literature regarding optimal vagus nerve stimulation. Current intensity should not exceed 5 mA, as stated by International Electrotechnical Commission(IEC) standards.
