Market Analysis
Market Strategy
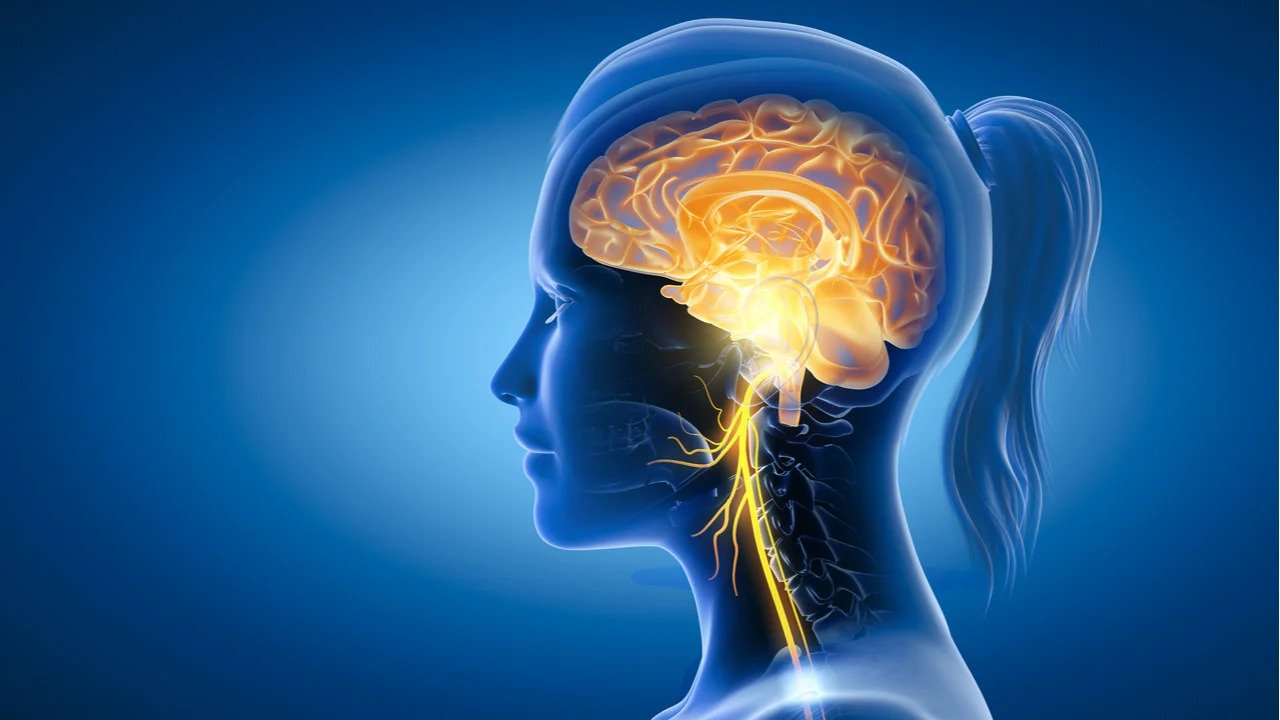
The market size for stroke therapy in the US is predicted to hit USD $65.45 billion by 2030, and the market size for Vagus Nerve Stimulation (VNS) devices in the US is $277.17 million, with an 18.0% compound annual growth rate. Our target market for stingray would be hospitals that intake and operate on the 795,000 adult stroke patients annually in the US.
Competition
Potency of Stimulation |
+ |
++ |
+ |
xx |
Ease of use for clinicians |
++ |
xx |
x |
+ |
Invasiveness |
++ |
xx |
++ |
++ |
Quality of Reading |
+ |
++ |
x |
x |
Currently, the field of VNS therapy includes the Vivistim implant, the ElectroCore cervical transcutaneous device, and the Soterix in-ear transcutaneous device. The key user needs include a high potency of stimulation, ease of use for the clinicians who will administer the device, low levels of invasiveness to minimize impact on the post-surgery patient, and a high quality of feedback from the nerve. While ElectroCore and Soterix are non-invasive, they lack the feedback loop and FDA approval for stroke patients. Vivistim implant is currently the only VNS therapy device in the market, for stroke patients, that has a FDA approval. However, it lacks a feedback loop and costs upward of $10,000, while Stingray will be sold for only $450. As the table shows, each device has its advantages and disadvantages, but no one device is able to meet all user needs, driving our team’s motivation to develop Stingray.